“Ina Vernikouskaya1,2, Axel Bornstedt1, Volker Rasche1,2 1 Department of Internal Medicine II, University Hospital of Ulm, Ulm, Germany 2 Small Animal MRI, Medical Faculty, University of Ulm, Ulm, Germany”
Cryogenically cooled resonators (CCRs) are a recent addition to small animal imaging applications. CCR use has provided substantial gains in SNR, making them a valuable tool even for high-field small animal MRI.
This study investigates their potential for imaging at 500MHz, in particular testing high-resolution brain imaging and functional cardiac imaging.
CCR application has shown that scan time can be reduced by 75% without sacrificing image fidelity, or that the spatial resolution can be doubled without sacrificing scan time. Examples will be shown for high-resolution morphologic brain imaging and volumetric functional cardiac imaging at isotropic spatial resolution.
Introduction
Noise in the final MR image is mainly dominated by two factors – RF emission due to the thermally driven Brownian motion of electrons within the body’s conducting tissue, and electronic noise introduced by the whole receive chain of the MR scanner (receive coil, electronics, etc.).
At mid and high fields, usually the patient is the dominant noise source. But in small animal MRI the imaged volumes being measured are of a size that renders the sample noise contribution comparable or even smaller than the thermal noise contributions of the receiver system, even at high magnetic fields. Thus reducing thermal noise significantly increases overall SNR [1].
Cooling of RF probes was an early idea in NMR, but recently, CCRs have been applied to in vivo small animal imaging realizing substantial SNR improvements by up to a factor of 4 compared to conventional room- temperature probes [2, 3], even higher for dedicated applications such as rapid cardiac imaging [4].
According to theory, the possible gain in SNR for CCRs scales inversely with the field strength. However, for small resonators as e.g. applied for mouse brain imaging – even at 500MHz – significant SNR gains are expected, enabling faster imaging or higher spatial resolutions.
Methods and Materials
Animal Preparation
Measurements on three wild-type mice (C57/B6) were carried out under isoflurane (3% for induction and ~1.5% for maintenance) anesthesia, with physiology monitored by an MR compatible small animal monitoring system.
With breathing frequency maintained between 50-65 cycles per minute, and body temperature controlled, the mice were prone or supine depending upon the probe in use.
Acquisition Protocol
Using a BioSpec 117/16 system, all data acquisition protocols were performed twice, each with data reception by a dedicated 2-element CCR brain transmit/receive array coil and a dedicated 4-element brain/heart receive array coil. For receive-only coils, a quadrature volume resonator (TO72, Bruker BioSpin) was used for excitation.
Brain MRI Protocol
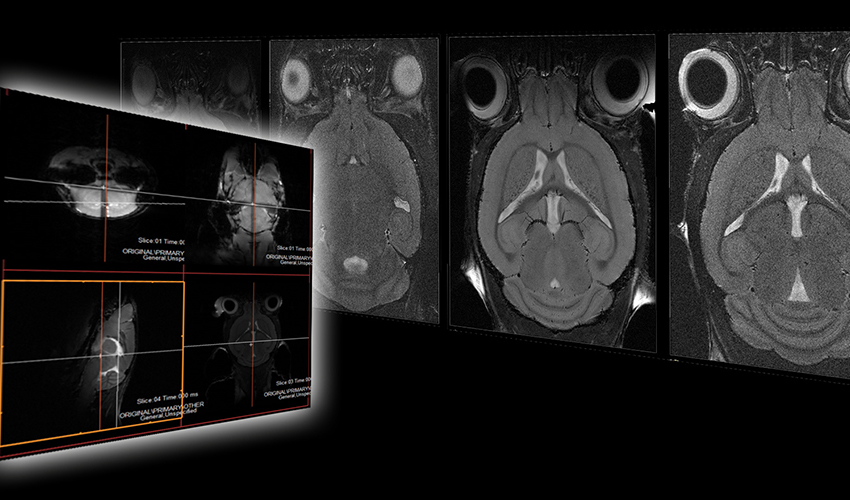
Para-coronal slices were acquired. To ensure reproducible acquisition geometry, the image plane was aligned with the mid-sagittal line (rotation around AP axis) and the cortex (rotation around the FH axis) as shown in Fig 1.
No rotation around the RL axis was performed to minimize B1-induced intensity modulation across the image. For CCR acquisitions, the reference gain was optimized in the central slice of the imaging stack, the extent of which was kept below 3mm to avoid substantial B1–variation over its extension.
All SNR comparisons were performed with mildly T2-weighted RARE imaging sequences (see Table 1 for sequence details).
Sequences included a low resolution (BLR) scan with different numbers of excitation (NEX), a low resolution scan with reduced slice thickness (BMR), and a high-resolution scan (BHR).
Cardiac MRI Protocol
The evaluation of the performance for cardiac applications was based on 2D and 3D cine acquisitions. For 2D acquisitions (CINE2D), cine images were acquired in 2 chamber, 4 chamber and short axis geometry (Fig. 2).
For 3D cine imaging (CINE3D), the 3D-volume was planned in para-coronal geometry (Fig. 3).
The volume was aligned with the long axis of the heart. The great vessels were thoroughly excluded from the excitation volume to minimize blood saturation ensuring sufficient blood-myocardium contrast.
All data was reconstructed using a self-gated reconstruction technique (IntraGate, Bruker Biospin). Refer to Table 2 for sequence details.
Data Analysis
Signal-to-noise ratios (SNR) were calculated according to:

with IROI being the mean value of a manually drawn region of interest, IBG the mean background signal, and σBG the standard deviation of the background signal.
For the brain data the average SNR of 4 segments of the left and right hemisphere was used for SNR comparisons.
For the cardiac data, the mean SNR was calculated independently for the anterior, septal, lateral and posterior wall. SNR comparisons were restricted to the 2D acquisitions, becuase, in 3D, parallel imaging was used.
Statistical relevance of the results was analyzed by a paired Student’s T-test assuming a heteroscedastic variance of the two samples. All ROIs were chosen at similar locations to ensure a fair comparison of the results.
Results
Brain Morphology
Direct comparison of the images acquired with the different investigated coils and imaging protocols reveals a significant increase of SNR with the CryoCoil (bottom row) as shown in Fig. 4.

Figure 4: 2D RARE images of murine brain acquired with 4-element brain array coil (top row) and CryoProbe (bottom row): a) 65×65µm2, slice thickness 0.5mm, NEX=4; b) 65×65µm2, slice thickness 0.5mm, NEX=1; c) 65×65µm2, slice thickness 0.25mm, NEX=4; d) 35x35x200 µm3, NEX=5; 65×65µm2, slice thickness 0.5mm, NEX=1; f) 65×65µm2, slice thickness 0.25mm, NEX=1; g) 65×65µm2, slice thickness 0.25mm, NEX=4; h) 35x35x200 µm3, NEX=5.
The respective SNR analysis clearly shows a more than threefold gain in SNR for all investigated protocols (Fig. 5, top). All improvements were highly significant (p-value < 0.01).
Without affecting the SNR, either the acquisition time could be reduced by 75% at constant spatial resolution, or the spatial resolution could be improved by a factor of two at constant acquisition time (Fig. 4).
Utilizing the SNR gain, the CryoCoil enabled high-resolution imaging of the brain in acceptable acquisition times (Table 1, Fig. 6).

Figure 6: Direct comparison of the cryogenic coil (a,c) and the 4-element phased array coil (b,d) in high resolution brain imaging.
Cardiac Function
In reconstructed SNR maps, a clear increase in the SNR can be appreciated in all regions of the myocardium (Fig. 7).
The mean gain in SNR (Fig. 5, bottom) with the CCR was 2.8, with individual improvements of 2.65 (anterior), 2.75 (septal), 2.85 (lateral) and 2.95 (posterior). All improvements were highly significant (p-value < 0.01). Interestingly, the strongest gain was obtained in the posterior region of the myocardium indicating a more homogeneous sensitivity profile of the 2-element coil, which can also be appreciated in the 2-chamber view (Fig. 8).

Figure 8: 4-(left) and 2-(right) chamber view acquired with the CryoProbe (top) and the 4-element cardiac coil (bottom).
Reconstruction of the 3D CINE data yielded sufficient image quality and contrast for the evaluation of the contractile motion (Fig. 9). Mean CNR/SNR resulted in 0.46/7.5 for 2D and 0.29/9.8 for 3D. From the isotropic 3D data the conventional 2-chamber, 4-chamber and short axis views could be retrieved by multi-planar reformatting (Fig. 9).

Figure 9: Isotropic CINE data acquired with a 2-element CryoProbe (top); comparison of 3D iso (1503 µm3) reformats with conventionally acquired (100x100x500 µm3) equatorial short axis and 2/4-chamber views (bottom).
Conclusions
In this study, it could be demonstrated that even at 11.7T a significant improvement in SNR can be obtained through the application of a CCR. In direct comparison to four-element room temperature surface coils, significant SNR gains in the order of 3 were obtained in both brain and cardiac imaging.
Surprisingly, even for rather deep-lying structures such as the posterior sections of the myocardium, a clear gain in SNR could still be observed by the CCR. This clearly indicates a less severe drop in B1, with increasing depth, than expected. The gain in SNR facilitates rapid imaging at high spatial resolution. For conventional imaging applications, the primary gain in applying CCRs is the significantly reduced scan times.
Considering the high gain in SNR, acquisition times can be reduced by 75% without sacrificing image quality when compared to the conventional room-temperature coil approach. This may become of special interest for high volume imaging sites for substantial increase of throughput. Yet more relevant may be the potentially higher spatial resolution that the CCR technology enables.
For a variety of brain applications, compromises between spatial resolution and data acquisition times have to be made. With the application of CCRs, this compromise may be shifted substantially towards higher spatial resolution.
In diffusion tensor imaging (DTE) in vivo acquisition times in the order of 110 minutes have been reported for a decent coverage of the murine brain with sufficient diffusion directions. By using the CCRs, the acquisition time for a similar acquisition protocol can be reduced to about 30 minutes, while simultaneously improving the spatial resolution by a factor of 2 [5, 6]. Similar gains can be expected for dedicated applications demanding high spatial resolution, such as the direct visualization of cellular layers in the mouse brain [7], where the shown imaging protocols indicate the potential for substantial scan time reduction without any sacrifice in spatial resolution.
Wagenhaus et al. [4] showed the benefit of high-resolution cine imaging using CCRs at 9.4T. With the suggested 3D imaging protocol facilitating isotropic spatial resolution, cardiac imaging in the mouse may become less dependent on the scan geometry planning and the subsequent selection of the analysis planes may further reduce user dependability of the results.
In conclusion, the markedly improved SNR of the CCR will render higher throughput with conventional scan protocols or facilitate high-resolution, high-quality scanning thereby enabling new applications.
Visit the University Hospital of Ulm’s website: www.uniklinik-ulm.de
References:
1. Darrasse, L. and J.C. Ginefri, Perspectives with cryogenic RF probes in biomedical MRI. Biochimie, 2003. 85(9): p. 915-37.
2. Baltes, C., et al., Micro MRI of the mouse brain usinga novel 400 MHz cryogenic quadrature RF probe. NMR Biomed, 2009. 22(8): p. 834-42.
3. Ratering, D., et al., Performance of a 200 -MHz cryogenic RF probe designed for MRI and MRS of the murine brain.
4. Wagenhaus, B., et al., Functional and morphological cardiac magnetic resonance imaging of mice using a cryogenic quadrature radiofrequency coil. PLoS One, 2012. 7(8): p. e42383.
5. Harsan, L.A., et al., In vivo diffusion tensor magnetic resonance imaging and fiber tracking of the mouse brain. NMR Biomed, 2010. 23(7): p. 884-96.
6. Mueller, H.P., et al., Fast diffusion tensor magnetic resonance imaging of the mouse brain at ultrahigh-field: aiming at cohort studies. PLoS One, 2013(revised).
7. Boretius, S., et al., MRI of cellular layers in mouse brain in vivo. Neuroimage, 2009. 47(4): p. 1252- 60.