Applications for in vivo fluorescent small animal imaging are varied and include cell tracking, tracer/probe development, and detection of molecular biomarkers. These methods have been applied in models of cancer, infection, inflammation, and stem cell biology. The catalogue of fluorescent reagents has expanded significantly in the past decade (Table 1). There have been two primary developments in the evolution of in vivo fluorescent reagents during this time: 1) red-shifted fluorescent proteins (e.g. tdTomato) and NIR dyes (e.g. Cy7) are more commonly used for in vivo imaging applications, and 2) the range of validated targeted probes has expanded significantly. Red-shifted light traverses tissue with less light scatter relative to photons in the blue/green spectrum. As a result, redshifted fluorescent reporters provide significantly improved sensitivity for in vivo small animal imaging applications.

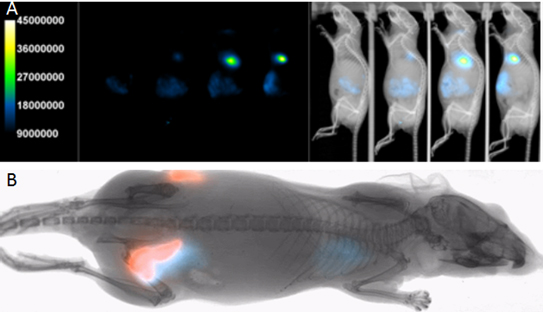
Several red-shifted fluorescent protein variants have been developed and genetic cell and animal reporter models have subsequently been generated (Fig. 1). For example, Ullrich et al. (2014) recently validated a MPC-mCherry tumor model, showing a good correlation in fluorescent intensity and tumor volume. This tumor model was subsequently employed in a dual fluorescence/radioisotope imaging study validating a novel theranostic agent (Ullrich et al., 2016). Fluorescent protein variants can differ not only in the relative fluorescent spectrum, but in fluorescent brightness. Kinnear et al. (2015) compared several red-shifted fluorescent proteins in a DNA vaccine model. Interestingly, tdTomato was found to provide better sensitivity compared to mCherry, Katushka, and Katushka2, despite a relatively lower red-shift of the tdTomato peak emission. There several possible explanations for this difference, but one might be that tdTomato is less susceptible to protease degradation.
Cell labeling using fluorescent NIR lipophilic dyes (e.g. DiR), or dyes that undergo endocytosis (e.g. QDots), are useful for short term cell tracking. Davison et al. (2013a) reported on a protocol using in vivo optical imaging and DiR pre-labeling to confirm MDA-MB-231 pulmonary implantation in a classic lung metastasis model. This non-invasive imaging protocol was subsequently employed in studies investigating the association of catalase and carcinoma associated fibroblasts in resulting tumor burden (Davison et al., 2013b; Weigel et al., 2014). These techniques are particularly beneficial where genetic manipulation is not possible or desirable, and has been applied in stem cell tracking, infection tracking, and in tracking autologous labeled immune cells (Eisenblätter et al., 2009).
Further, the range of validated commercial (and research) targeted NIR probes has expanded significantly in the past decade. These include specific molecular probes for tumors (Sasser et al., 2014), infectious agents (Sasser et al., 2013), inflammation (Robin-Jagerschmidt et al., 2016a), apoptosis (Robin-Jagerschmidt et al., 2016b), and angiogenesis (Edwards et al., 2009, Ke et al., 2012). Activatable fluorescent probes offer molecular detections with improved Signal-toNoise-Ratios (SNR). These “smart probes” often consist of a specific protease peptide recognition sequence flanked with auto-quenching NIR dyes that fluoresce only when the probe is cleaved, typically when reaching a target at a dedicated location (e.g. site of tumor, inflammation or injury) with high protease activities (Mahmood et al., 2003). In vivo validation for the protease probes has been performed in a range of models
Bruker Optical/X-ray imaging systems are equipped standard with full multispectral (Yudina et al., 2016) fluorescence VISNIR imaging capability, compatible with most custom and commercial reporters (Fig. 1). Bruker Optical/X-ray imaging systems have been employed with a range of fluorescent reporters including but not limited to YFP, eGFP, mCherry, TdTomato, IR650, IR 700, IR750, IR800, Alexa680, Alexa700, Alexa 750, Cy5, Cy5.5, Cy7, DiR, Qdots, and many others.
There are now several commercial suppliers of fluorescent reporters. Reporter constructs differ relative to application and price. For example, constructs differ in the fluorescent protein variant type (e.g. GFP, mCherry, tdTomato), the promoter, and often include complementary expression constructs. Imanis (www.imanislife.com) supplies dual reporters that combine fluorescent constructs with PET/SPECT and bioluminescence genetic reporters, providing bridge between in vivo optical, PET/SPECT, and even ex vivo fluorescence microscopy.

References
Ullrich M, Bergmann R, Peitzsch M, Cartellieri M, Qin N, Ehrhart-Bornstein M, Block NL, Schally AV, Pietzsch J, Eisenhofer G, Bornstein SR, Ziegler CG. (2014) in vivo fluorescence imaging and urinary monoamines as surrogate biomarkers of disease progression in a mouse model of pheochromocytoma. Endocrinology. 155(11):4149-56. doi: 10.1210/en.2014-1431.
Ullrich M, Bergmann R, Peitzsch M, Zenker EF, Cartellieri M, Bachmann M, Ehrhart-Bornstein M, Block NL, Schally AV, Eisenhofer G, Bornstein SR, Pietzsch J, Ziegler CG. Multimodal Somatostatin Receptor Theranostics Using [(64)Cu]Cu-/[(177)Lu]Lu-DOTA-(Tyr(3))octreotate and AN-238 in a Mouse Pheochromocytoma Model. Theranostics. 2016 Mar 10;6(5):650-65. doi: 10.7150/thno.14479.
Kinnear E, Caproni LJ, Tregoning JS. (2015) A Comparison of Red Fluorescent Proteins to Model DNA Vaccine Expression by Whole Animal in vivo Imaging. PLoS One. 19;10(6):e0130375. doi: 10.1371/ journal.pone.0130375.
Davison CA, Chapman SE, Sasser TA, Wathen C, Diener J, Schafer ZT, Leevy WM. (2013a) Multimodal optical, X-ray CT, and SPECT imaging of a mouse model of breast cancer lung metastasis. Curr Mol Med. 13(3):368-76.
Davison CA, Durbin SM, Thau MR, Zellmer VR, Chapman SE, Diener J, Wathen C, Leevy WM, Schafer ZT. (2013b) Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res. 15;73(12):3704-15. doi: 10.1158/0008-5472.CAN-12-2482.
Weigel KJ, Jakimenko A, Conti BA, Chapman SE, Kaliney WJ, Leevy WM, Champion MM, Schafer ZT. (2014) CAF-secreted IGFBPs regulate breast cancer cell anoikis. Mol Cancer Res. 12(6):855-66. doi: 10.1158/1541-7786.MCR-14-0090.
Eisenblätter M, Ehrchen J, Varga G, Sunderkötter C, Heindel W, Roth J, Bremer C, Wall A. in vivo optical imaging of cellular inflammatory response in granuloma formation using fluorescence-labeled macrophages. J Nucl Med. 2009 Oct;50(10):1676-82. doi: 10.2967/jnumed.108.060707.
Sasser TA, Van Praagh A, Waldeck (2014) Preclinical oncology studies using multimodal optical imaging. https://www.bruker.com/fileadmin/user_upload/8-PDF-Docs/PreclinicalImaging/Brochures/Optical_ Oncology_Review_apps_T154170.pdf
Sasser TA, Van Avermaete AE, White A, Chapman S, Johnson JR, Van Avermaete T, Gammon ST, Leevy WM. (2013) Bacterial infection probes and imaging strategies in clinical nuclear medicine and preclinical molecular imaging. Curr Top Med Chem. 13(4):479-87.
Robin-Jagerschmidt, C, Berrocal E, Ceccotti MC, Auberval M, Yudina A, Waldeck J (2016a) NonInvasive Near-infrared fluorescence imaging of neutrophil infiltration in a mouse model of acute lung inflammation. https://www.bruker.com/fileadmin/user_upload/8-PDF-Docs/PreclinicalImaging/Application_Notes/ T160683_Lung-Inflammation-lores.pdf
Robin-Jagerschmidt, C, Berrocal E, Ceccotti MC, Auberval M, Yudina A, Waldeck J (2016b) Simultaneous non-Invasive in vivo Imaging of apoptosis and angiogenesis in a mouse MDA-MB-231 xenograft model. https://www.bruker.com/fileadmin/user_upload/8-PDF-Docs/PreclinicalImaging/Application_Notes/ T160682__Simultaneous-Apoptosis-Angiogenesis.pdf © Bruker BioSpin 01/17 T163372 Bruker BioSpin info@bruker.com www.bruker.com
Edwards WB, Akers WJ, Ye Y, Cheney PP, Bloch S, Xu B, Laforest R, Achilefu S. (2009) Multimodal imaging of integrin receptor-positive tumors by bioluminescence, fluorescence, gamma scintigraphy, and single-photon emission computed tomography using a cyclic RGD peptide labeled with a near-infrared fluorescent dye and a radionuclide. Mol Imaging. 8(2):101-10.
Ke S, Zhang F, Wang W, Qiu X, Lin J, Cameron AG, Zou C, Gao X, Zou C, Zhu VF, Li M. (2012) Multiple target-specific molecular imaging agents detect liver cancer in a preclinical model. Curr Mol Med. 12(8):944-51.
Mahmood U, Weissleder R. (2003) Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther. 2(5):489-96.
Yudina A, Sasser TA (2016) in vivo multispectral fluorescent imaging. https://www.bruker.com/fileadmin/user_upload/8-PDF-Docs/PreclinicalImaging/Application_Notes/ Multispectral_Fluorescent_Imaging_appnote_T161828.pdf